Research
The Wolf group is dedicated to discovering new highly reactive transition metal compounds and exploring their applications in coordination chemistry and homogeneous catalysis. Our research primarily focuses on the chemistry of abundant and biorelevant 3d-metal complexes, particularly iron, cobalt, and nickel, often in very low oxidation states. We also investigate the remarkable ion-pairing and aggregation effects on the reactivity of low-valent transition metalate salts.
Central to many of our projects is phosphorus chemistry, especially the intriguing and complex transformations of the white phosphorus (P4) molecule. One of our primary objectives is to develop new methods for synthesizing useful organophosphorus compounds directly from P4. Additionally, we utilize phosphaalkynes and phosphabenzenes as building blocks to create new versatile phosphaorganic and phosphaorganometallic complexes.
Photoredox catalysis represents another significant area of our research, where we concentrate on developing 3d transition metal catalysts in various visible-light-mediated transformations. For more information about our different projects, please explore the tabs below.
Research
-
Phosphorus Functionalization
-
Phosphorus Compounds
-
Transition Metalate Anions
-
Photocatalysis
White Phosphorus Activation
Phosphorus Functionalization:
Organophosphorus compounds (OPCs) are a very important and industrially relevant class of substances that have a wide range of applications. They are utilized in organic synthesis, catalysis, as flame retardants, and photoinitiators. However, most of these valuable compounds are currently produced through inefficient, multi-step processes. This typically involves oxidizing white phosphorus (P4) with toxic chlorine gas to create chlorinated intermediates, notably PCl3. The subsequent formation of products through salt metathesis generates large amounts of undesirable chlorine-containing waste. There is an urgent need for more efficient and environmentally friendly synthetic methods, yet direct and catalytic methods for converting P4 into OPCs are still largely unexplored.
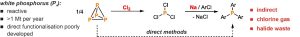
We are exploring new methods for the activation and functionalization of white phosphorus. A key objective of our research is to develop new metal-mediated transformations of P4 into organophosphorus compounds. To achieve this, we are designing and synthesizing novel transition metal compounds that can generate reactive phosphorus units.
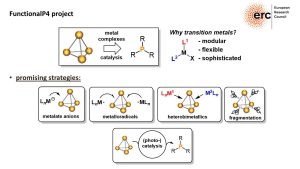
We develop the concept of heterobimetallic P4 activation, in which two electronically different metal complexes interact cooperatively with P4. The reactions of the phosphorus fragments in these new reactive complexes with electrophiles yield novel and fundamentally interesting organophosphorus compounds.
Developing catalytic methods for the functionalization of P4 is of primary importance. Our goal is to create innovative and synthetically useful catalytic procedures for converting P4 into organophosphorus compounds. This work aims to significantly advance the field of phosphorus chemistry and contribute to the development of more sustainable synthesis methods.
Our research in organophosphorus and phosphaorganometallic chemistry is inspired by the diagonal relationship between phosphorus and carbon in the periodic table. Introducing P atoms into the scaffold of organic compounds and ligands significantly affects the electronic structures of the molecules, leading to changes in the properties of organometallic compounds. Ongoing projects specifically focus on phosphabenzenes and phosphaalkynes, which are used to synthesize compounds with fascinating new structures and properties.
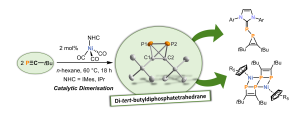
Phosphatetrahedranes
Tetrahedranes are a class of reactive organic molecules consisting of four carbon atoms arranged in a tetrahedral configuration. They are highly reactive due to the significant bond strain present in their structure. Until recently, neutral tetrahedranes with heteroatoms in the backbone were not well understood. In 2019, our research group published the synthesis of di-tert-butyldiphosphatetrahedrane, (tBuCP)₂, which is the first tetrahedrane featuring both phosphorus and carbon in its tetrahedral backbone. Almost simultaneously, in 2020, Cummins and co-workers described the synthesis of tri-tert-butylphosphatetrahedrane, (tBuC)₃P. The discovery of these first phosphatetrahedranes marked the beginning of a previously unexplored area of research within organophosphorus chemistry.
In this project, we are conducting fundamental and comprehensive research into the synthesis, chemical properties, and reactivity of phosphatetrahedranes. So far, we have elucidated the molecular and electronic structure of our di-tert-butyldiphosphatetrahedrane, (tBuCP)₂. Additionally, we have gained valuable insights into the reaction patterns of this compound, which has become a useful synthetic building block. Ongoing work focuses on the isolation of new phosphatetrahedranes and fundamental studies on their reactivity with electrophiles, nucleophiles, and reducing agents, as well as their coordination chemistry with transition metals.
Angew. Chem. Int. Ed. 2019, 58, 16918–16922; Chem. Commun. 2021, 57, 2356–2359; Angew. Chem. Int. Ed.2021, 60, 6435–6440; Chem. Eur. J. 2021, 27, 14936–14946; Z. Anorg. Allg. Chem. 2022, 648, e202200124; Chem. Sci. 2024, 15, 5596–5603; Dalton Trans. 2024, 53, 10113–10119.
Phosphinines: Coordination Chemistry, Small Molecule Activation and Catalytic Applications
In collaboration with Prof. Christian Müller’s group at FU Berlin, we are developing new coordination compounds of phosphabenzenes (also known as phosphinines). These complexes can activate a variety of small molecules, including the typically unreactive CO2. Additionally, we explore the usefulness of phosphinine complexes and phosphacyclohexadienyl anions in homogeneous catalysis, particularly in hydrofunctionalization reactions involving alkenes, ketones, and imines.
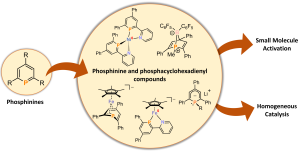
Organometallics 2015, 34, 622-635; Dalton Trans. 2016, 45, 8875–8884; Eur. J. Inorg. Chem. 2019, 1567–1574; Chem. Eur. J. 2020, 26, 7788–7800; Angew. Chem. Int. Ed. 2019, 58, 15407–15411; Inorg. Chem.2020, 59, 9951–9961; Coord. Chem. Rev. 2021, 432, 213927.
Low-Valent Transition Metalate Anions
Low-valent alkene and polyarene complexes serve as sources of transition metal anion synthons for unusual new compounds and novel catalysts. By starting with anionic synthons, we create “functional” anionic complexes, metal clusters, and oligonuclear complexes. We also investigate the catalytic properties of these complexes in reductive transformations. Our research primarily focuses on readily available and relatively inexpensive 3d transition metals, specifically iron and cobalt.
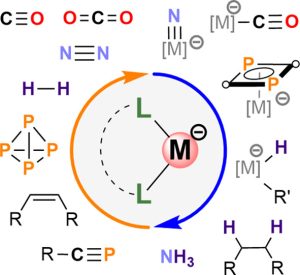
Chem. Rev. 2024, 124, 1323–1463; J. Am. Chem. Soc. 2025, 147, 7083–7093.
DFG Research Training Group 2620 Ion Pairs in Molecular Reactivity (project P10)
Ion-Pairing and Aggregation Effects in Transition Metal Hydride Intermediates
Within the graduate school Ion Pairs in Molecular Reactivity, we study the impact of ion-pairing and aggregation on catalytically relevant transition metal hydride anions. The project focuses on cobalt and iron hydrides, which are crucial intermediates in catalytic alkene hydrogenations. Our previous work has shown that the coordination of Li+ and Mg2+ cations to hydride ligands causes a significant rate acceleration in cobalt-catalyzed alkene hydrogenation. Related studies of ammonia borane dehydrogenation revealed second-order kinetics for the catalyst, suggesting that binuclear intermediates may be important. Some dinuclear hydridocobaltate salts have been isolated and structurally characterized, but the mechanistic role of this dimerization process is presently unclear.
Our project systematically investigates the role of ion-pairing and aggregation in anionic transition metal hydrides. Furthermore, we explore the use of chiral (magnesium) cations with (achiral) anionic catalysts for asymmetric hydrofunctionalisation reactions. The investications are performed in close collaboration with other groups within the RTG.
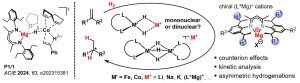
Selected publications: Chem. Eur. J. 2019, 25, 238–245; ACS Catal. 2019, 9, 7596–7606; Angew. Chem. Int. Ed. 2024, 63, e202315381.
Further information on the RTG: https://www.uni-regensburg.de/chemistry-pharmacy/grk-2620/home/index.html
Applications (single .pdf document) should typically include a letter of motivation, a CV, an academic transcript of records, and contact information of two references, preferably in English. Prospective PhD students interested in the Research Training Group Ion Pairs in Reaction should apply exclusively to rtg-ion-pairs@uni-regensburg.de
Collaborative Research Center (CRC) 325 Assembly-Controlled Photocatalysis (project A4)
In collaboration with research groups in Regensburg, Munich, and Leipzig, we are developing photoredox reactions that are catalyzed by 3D transition metal complexes. By utilizing substrate-catalyst preassembly, we aim to address some of the key limitations of current 3d metal photocatalysts, particularly the very short excited-state lifetimes of most 3d metal species.
This project explores two types of interactions: ‘outer sphere’ interactions, where the substrate interacts with the ligand sphere of the metal (for example, through π-π interactions with heterocyclic ligands or hydrogen bonding with pendant hydrogen bond donors), and ‘inner sphere’ interactions, where the substrate binds strongly or weakly to the metal atom.
In both scenarios, coordination chemistry studies identify the optimal design principles for the desired photocatalysts, along with an analysis of their electronic structures. To effectively guide the development of new photocatalysts, it is essential to understand the specific interactions that occur between substrates and catalysts in both the ground and excited states. This foundational knowledge enables the targeted development of applications in organic synthesis, which leverage the unique advantages of the developed catalysts compared to precious metal compounds.
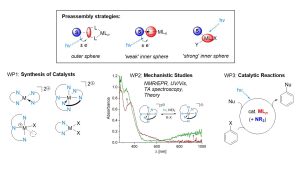
Chem. Sci. 2025, 16, 2751–2762; Angew. Chem. Int. Ed. 2024, 63, e202405780.
Further information on the CRC can be found through the following link: https://go.ur.de/crc325
Prospective PhD students interested in the CRC Assembly-Controlled Photocatalysis should apply exclusively to apply-crc325@ur.de. Applications (single .pdf document) should typically include a letter of motivation, CV, an academic transcript of records, and contact information of two references, preferably in English.
